曲妥珠单抗与帕妥珠单抗双靶向联合化疗在人表皮生长因子受体2阳性乳腺癌中的应用效果
作者: 蒋海英
【摘要】 目的 探讨曲妥珠单抗与帕妥珠单抗双靶向联合化疗药物治疗人表皮生长因子受体2(HER-2)阳性乳腺癌的临床效果。方法 选择2018年3月- 2023年6月六盘水市人民医院收治的72例HER-2阳性乳腺癌患者,根据组间基线资料均衡可比的原则,按随机数字表法分成两组,每组36例。两组患者均采取多西他赛联合卡铂治疗,在此基础上对照组加曲妥珠单抗治疗7个疗程,观察组则应用曲妥珠单抗与帕妥珠单抗双靶向联合治疗7个疗程。对比两组患者的临床疗效、治疗前后血清指标[糖类抗原125(CA-125)、糖类抗原153(CA-153)]水平及治疗期间不良反应情况。结果 观察组患者的治疗有效率(80.55%)显著高于对照组(50.00%),组间差异有统计学意义(P<0.05)。治疗前,两组患者CA-125、CA-153血清指标水平比较,差异均无统计学意义(P>0.05);治疗7个疗程后,两组患者CA-125、CA-153血清指标水平均降低,但观察组低于对照组,差异有统计学意义(P<0.05)。治疗期间,对照组患者的不良反应发生率为11.11%,低于观察组的13.89%,但组间差异无统计学意义(P>0.05)。结论 对于HER-2阳性乳腺癌,采取曲妥珠单抗与帕妥珠单抗双靶向联合化疗药物治法,治疗效果好,显著降低血清CA-125、CA-153水平,同时不增加不良反应发生率。
【关键词】 人表皮生长因子受体2;乳腺癌;化疗;曲妥珠单抗;帕妥珠单抗
中图分类号 R737.9 文献标识码 A 文章编号 1671-0223(2024)13--03
Application of trastuzumab and parstuzumab double targeted chemotherapy in human epidermal growth factor receptor 2 positive breast cancer Jiang Haiying. Department of Oncology and Hematology, Liupanshui People's Hospital, Liupanshui 553000, China
【Abstract】 Objective To investigate the clinical effect of trastuzumab and patouzumab combined with chemotherapy in the treatment of human epidermal growth factor receptor 2 (HER-2) positive breast cancer. Methods A total of 72 HER-2 positive breast cancer patients admitted to Liupanshui People's Hospital from March 2018 to June 2023 were selected. According to the principle of balanced and comparable baseline data between groups, they were divided into two groups by random number table, with 36 cases in each group. Both groups of patients were treated with docetaxel combined with carboplatin. On this basis, the control group was treated with trastuzumab for 7 courses, while the observation group was treated with a dual targeted combination of trastuzumab and pertuzumab for 7 courses. Compare the clinical efficacy, serum levels of carbohydrate antigen 125 (CA-125) and carbohydrate antigen 153 (CA-153) before and after treatment, and adverse reactions during treatment between two groups of patients. Results The effective rate of treatment in the observation group (80.55%) was significantly higher than that in the control group (50.00%), and the difference between the groups was statistically significant (P<0.05). Before treatment, there was no statistically significant difference in the serum levels of CA-125 and CA-153 between the two groups of patients (P>0.05). After 7 courses of treatment, the serum levels of CA-125 and CA-153 in both groups of patients decreased, but the observation group was lower than the control group, and the difference was statistically significant (P<0.05). During the treatment period, the incidence of adverse reactions in the control group was 11.11%, lower than the observation group's 13.89%, but there was no statistically significant difference between the groups (P>0.05). Conclusion For HER-2 positive breast cancer, the dual targeted combination chemotherapy of trastuzumab and patouzumab has a good therapeutic effect, significantly reducing the levels of serum CA-125 and CA-153, while not increasing the incidence of adverse reactions.
【Key words】 Human epidermal growth factor receptor 2; Breast cancer; Chemotherapy; Trastuzumab; Patuzumab
乳腺癌是女性最常见的恶性肿瘤之一,好发于30~50岁人群[1]。在乳腺癌群体中,10%~20%的患者表现为人表皮生长因子受体2(HER-2)阳性,HER-2阳性乳腺癌患者的恶性程度高,常表现出较差的预后[2]。而因为乳腺癌的早期症状不典型,所以一旦确诊往往处在中晚期阶段,此时患者失去手术治疗机会,临床中多是采取放化疗以控制患者病情以及延长患者生存时间[3]。在对HER-2阳性乳腺癌治疗上,应用具备抗HER-2作用的药物同化疗药物联合是有效的治疗手段,提高治疗的效果及改善患者预后。曲妥珠单抗作为抗HER-2药物,能够改善患者病理症状,延长患者生存时间,而在长期研究中也发现部分患者存在耐药问题,影响了一些患者的治疗效果[4]。帕妥珠单抗则属于第2代抗HER-2药物,相较于单纯曲妥珠单抗治疗,采取曲妥珠单抗与帕妥珠单抗双联靶向干预,能够起到协同配合作用,实现双重阻断HER-2的作用,促进患者病情的改善,提高临床治疗效果[5]。本研究针对HER-2阳性乳腺癌患者,探讨曲妥珠单抗与帕妥珠单抗双靶向联合化疗药物的临床效果。
1 对象与方法
1.1 研究对象
选择2018年3月- 2023年6月六盘水市人民医院收治的72例HER-2阳性乳腺癌患者。纳入标准:①参照《中国抗癌协会乳腺癌诊治指南与规范(2015版)》[6]对于乳腺癌的诊断标准,穿刺活检也证实病变,且经免疫组织化学检测确诊HER-2阳性;②无远处器官转移、具有可测量的病灶,预期生存时间>6个月。排除标准:①存在远处器官转移者;②肝肾等重要脏器损伤者;③对研究所用药物存在过敏反应者;④精神疾病或者认知障碍者。根据组间年龄、肿瘤直径、乳腺癌类型等基线资料均衡可比的原则,按随机数字表法分成两组,每组36例。观察组年龄31~68岁,平均49.15±5.56岁;肿瘤直径1.4~8.5cm,平均4.56±0.77cm;乳腺癌类型:浸润性癌22例,非浸润癌14例。对照组年龄30~68岁,平均48.92±5.68岁;肿瘤直径1.5~8.5cm,平均4.61±0.78cm;乳腺癌类型:浸润性癌24例,非浸润癌12例。两组患者上述基线资料比较,差异无统计意义(P>0.05)。研究通过医院伦理委员会批准,患者本人或者家属均签署知情同意书。
1.2 治疗方法
入组的患者均予以化疗干预,多西他赛注射液(江苏奥赛康药业股份有限公司,国药准字H20064301,规格0.5ml∶20mg)75mg/m2溶入250ml 0.9%氯化钠注射液,静脉滴注,1h内完成,化疗第1天给药。卡铂注射液(齐鲁制药有限公司,国药准字H20020180,规格10ml∶100mg),取曲线下面积(AUC)=6溶入250ml 5%葡萄糖注射液,静脉滴注,30~60min完成,化疗第1天给药,21d为1个化疗周期,治疗7个周期。
对照组在常规化疗基础上加注射用曲妥珠单抗[Roche Pharma(Switzerland)Ltd.批准文号:S20050008,规格440mg/瓶]治疗,初始给药量8mg/kg,静脉滴注并且在1.5h内滴注完成,21d为1个疗程,1个疗程给药1次。自第3个疗程后调整用量6mg/kg并维持4个疗程,总治疗7个疗程。观察组在对照组的基础上加帕妥珠单抗[Roche Pharma(Schweiz)Ltd,注册证号S20180029,规格:420mg(14ml)/瓶],初始用量840mg,药物融入氯化钠溶液静脉滴注,21d为1个疗程,1个疗程用药1次。于第2个疗程调整用量420mg,维持6个疗程,持续治疗7个疗程。
1.3 观察指标
(1)临床疗效。治疗结束后,持续观察2个月,使用实体瘤疗效评价标准(RECIST)[7]评估治疗效果。完全缓解(CR):所有癌症病灶都消失,没有新的病灶出现,而且癌症标志物在正常范围内,这种状态至少持续4周。部分缓解(PR):癌症病灶的最大直径总和减少至少30%,并且这种状态至少持续4周。疾病稳定(SD):癌症病灶的最大直径总和没有达到部分缓解的程度,但也没有增加到疾病进展的程度。疾病进展(PD):癌症病灶的最大直径总和至少增加20%,或者出现新的病灶。治疗总有效率=(完全缓解+部分缓解)例数/总例数×100%。
(2)血清肿瘤标志物指标。两组患者治疗前与治疗7个疗程,采集静脉血液4ml,经3000r/min速度持续离心10min分离血清。采取电化学发光法检测血清糖类抗原-125(CA-125)、糖类抗原-153(CA-153)指标水平。
(3)不良反应发生率:包括恶心、呕吐、贫血、口腔溃疡等不良反应。
1.4 数据分析方法
运用SPSS 21.0统计学软件分析处理数据,疗效与不良反应属于计数资料,计算百分率(%),组间率比较采用χ2检验;肿瘤标志物属于计量资料,用“均数±标准差”表示,组间均数比较采用t检验。以P<0.05为差异有统计学意义。
2 结果
2.1 两组患者临床疗效比较
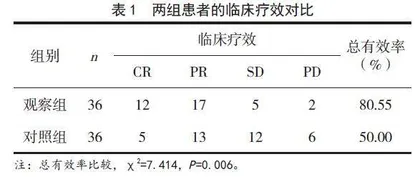
观察组患者的总有效率为80.55%,显著高于对照组患者的50.00%,差异有统计学意义(P<0.05),见表1。
2.2 两组患者肿瘤标志物指标比较
治疗前,两组患者CA-125、CA-153血清指标水平比较,差异无统计学意义(P>0.05);治疗7个疗程后,两组患者上述指标均降低,但观察组低于对照组,差异有统计学意义(P<0.05)。见表2。